1.)
b. sucrose
SHORT ANSWER
You might also consume a big bowl of pasta the night before a race because pasta is composed of carbohydrates which store the energy in your body for a long period of time, allowing more energy in the upcoming race.
'said ah life is BOOM chickawawa
Posted by winniboo at 3:36 AM 0 comments
ENZYMES ARE PROTEINS THAT SPEED UP SPECIFIC REACTIONS IN CELLS
-to start a chemical reaction, it if first necessary to weaken chemical bonds ion the reactants molecules
-Activation Energy: minimum amount of energy required to trigger a chemical reaction and activate the reactants

Posted by winniboo at 11:44 PM 0 comments

THE FUNCTIONS OF PROTEINS
- A PROTEIN is a polymer constructed from a set of just 20 kinds of monomers called amino acids
-proteins are responsible to day to day functioning of organisms
-proteins form structures such as hair, fur and make up muscles and provide long term nutrients storage
-proteins work in bloods, defending harmful substances, conveys messages from one cell to another, controls chemical reactions.
AMINO ACIDS
-Each AMINO ACID monomer consists of a central carbon atom bonded into four partners
-Three partners are the same in all amino acids
-One partner is a hydrogen acid
- the side group differs in each amino acid
-side group is responsible for properties of each amino acid such as if its is hydroxyl
BUILDING A PROTEIN
- Cell create proteins by linking amino acids together into a chain called POPYPEPTIDE
-Each link is created by a dehyrdation reaction between amino group of one amino acid and the carboxyl group of the next amino acid
-can arrange amino acids in different orders to make proteins
PROTEIN SHAPE
- a functional protein consists of one or more polypeptides precisely twisted, folded and coiled into a unique shape
-a protein's shape is also influenced by surrounding environment, generally water
-an unfavorable change in temperature, pH or some other quality of the environment can cause a protein to unravel and lose its shape.
-called DENATURATION
-like frying an egg is denaturation
 DENATURATION
DENATURATIONPosted by winniboo at 5:38 AM 0 comments
Characteristics of LIPIDS
-LIPIDS are a typical class of water-avoiding compounds such as Oil
- Water avoiding molecules are said to be HYDROPHOBIC
-Lipids acts as a boundary that surrounds and contains watery contents of your cell
- Common Lipids are like fats which store energy in your body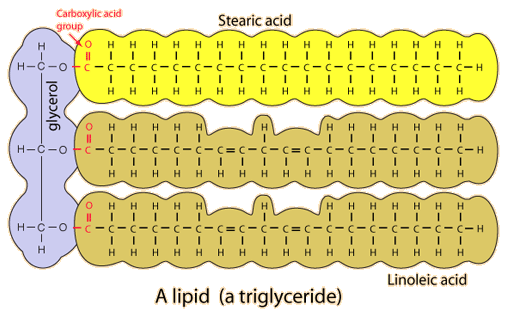
FATS
- FAT consists of three-carbon backbone called glycerol attached to three fatty acids, which contain long hydrocarbon chains
-Some fats are solid at room temperature while others fats, oils are liquid
- fatty cushion stores energy for later use and cushion your organs and provide your body with insulation
-SATURATED FAT: is a fat which all three fatty acid chains contain the maximum possible number of hydrogen atoms
-Saturated fat forms single bonds with others and hydrogen atoms, and are solid in room temp, butter
-UNSATURATED FAT: contains less than the maximum number of hydrogen atoms on one or more of its fatty acid chains, because some of its carbon atoms are double-bonded to each other
-unsaturated fat include fruits, veggies
-too many saturated fat can be unhealthy causing blood flow problems

Posted by winniboo at 5:09 AM 0 comments
 SUGAR / CARBOHYDRATE
SUGAR / CARBOHYDRATE
- an organic compound made up of sugar molecules.
- contain the elements: carbon, hydrogen and oxygen
-ratio: 1carbon, 2hydrogen 1 oxygen - these are a type of carbohydrates
- these are a type of carbohydrates
-WHEAT
-BREAD
MONOSACCHARIDES: 
- simple sugars containing just one sugar unit
-Examples: glucose, fructose, lactose
-many mono saccharides are found in sweet
things
-the right picture shows the complete molecular structure of glucose, sucrose and starch.
-glucose supply the fuel for cellular work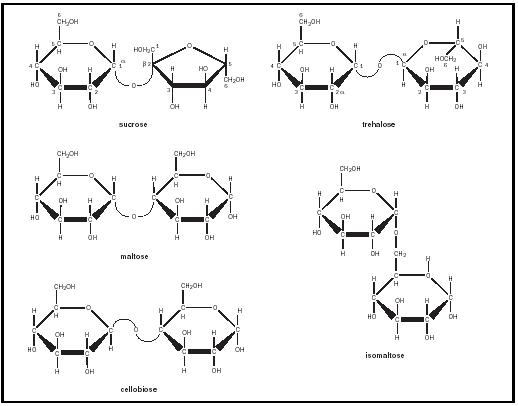 DISACCHARIDES:
DISACCHARIDES:
- constructed with two monosacchrides, double sugar using the dehydration reaction
- Sucrose is the most common disaccaride
- Sucrose consists of a glucose molecule linked to a fructose molecule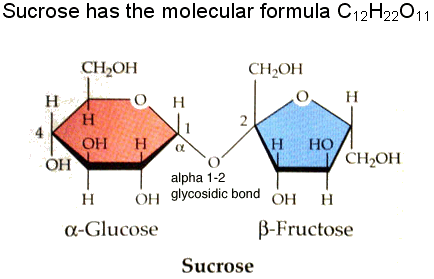 -Sap of maple trees are sucrose
-Sap of maple trees are sucrose
-Once consumed, sucrose can break down into glucose and fructose and used right away.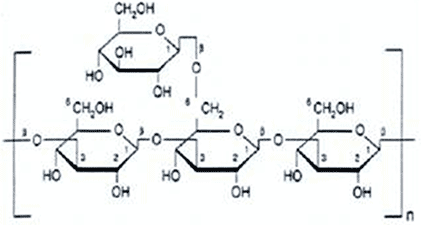 POLYSACCHARIDES:
POLYSACCHARIDES:
-Long polymer chains made up on simple sugar monomers
-complex carbohybrates
- starch is a polysaccharides, consists of glucose monomers
-Starches include: potatoes, rice and corn - glycogen is the excess sugar in the form of a polysaccharides
- glycogen is the excess sugar in the form of a polysaccharides
-
CELLUSE: serve as building materials
- almost all carbohydrates are hydrophillic
-however, celluse and starch and others don't dissolve in water
Concept Check:
1.Explain the difference between a monosaccharide and a disaccharide.Give an example of each. The difference between a monosaccharide and disaccharide is that a monosaccharide is a simple sugar with just one unit of sugar, while disaccharides are two monosaccharide together. A monosaccharide will be glucose and a dissaccharide will be sucrose, which is glucose and fructose added together.
2.Compare and contrast starch, glycogen, and cellulose. Starch, cellulose and glycogen are all made up of glucose monomers. Starch is a polysaccharides found in plants cells while glycogen is a excess sugary in form of polysaccharidesfound in animals such as turkeys. Cellulose on the other hand, serve as building materials to make fiber from plants.
3.How do animals store excess glucose molecules.Animals store excess glucose molecules in a form of glycogen since glycogen is the excess sugar found in animals.




Posted by winniboo at 3:33 AM 0 comments